I'd like to introduce a new Cinfony module,
Webel. Like the other components of
Cinfony, Webel implements a standard API (see for example, the
Pybel API) that covers a large proportion of common cheminformatics operations including reading/writing SMILES strings and InChIs, calculation of molecular weight and formula, molecular fingerprints, SMARTS searching, and descriptor calculation.
However, unlike the other components, Webel runs entirely off web services. All cheminformatics analysis is carried out using Rajarshi's
REST services (which use the
CDK and are hosted at Uppsala) and the NIH's
Chemical Identifier Resolver (by Markus Sitzmann, and which uses
Cactvs for much of its backend).
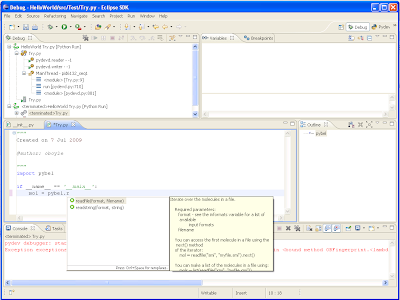
To use Webel, all you need to do is download
webel.py, and type "import webel" at a Python prompt (see example code below - it's basically the same as using Pybel if you're familiar with that).
So what are the advantages of running off webservices? First, as should be clear, there is the ease of installation. This means that Webel could easily be bundled in with some other software to provide some useful functionality. Second, Webel can still be used in environments where installation of a cheminformatics toolkit is simply not possible (
more on this next week!). Third, webservices may provide additional functionality not available elsewhere (e.g. the Chemical Resolver provides name-to-structure conversion as well as InChIKey resolution). Fourth, webservices are accessed across HTTP rather than through some type of language binding. As a result, Webel works equally well from CPython, Jython or IronPython. And finally, it's just a cool idea. :-)
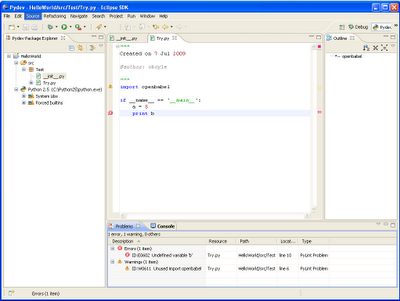
If you can think of any other advantages or potential applications, I'd be interested to hear them. In the meanwhile, here's some code that calculates the molecular weight of aspirin, its LogP, its InChI, gives alternate names for aspirin, and creates the PNG above:
import webel
mol = webel.readstring("name", "aspirin")
print "The molecular weight is %.1f" % mol.molwt
print "The InChI is %s" % mol.write("inchi")
print "LogP values are: %s" % mol.calcdesc(["ALOGPDescriptor"])
print "Aspirin is also known as: %s" % mol.write("names")
mol.draw(filename="aspirin.png", show=False)
...which gives...
C:\Tools\cinfony\trunk\cinfony>python example.py
The molecular weight is 180.2
The InChI is InChI=1/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12)
/f/h11H AuxInfo=1/1/N:5,3,4,1,2,12,6,7,11,9,8,10,13/E:(11,12)/F:5,3,4,1,2,12,6,7
,11,9,10,8,13/rA:21CCCCCCCOOOCCOHHHHHHHH/rB:;a1;a2a3;;a1;a2a6;;;;s6d8s10;s5d9;s7
s12;s10;s1;s2;s3;s4;s5;s5;s5;/rC:6.3301,-.56,0;4.5981,-1.56,0;6.3301,-1.56,0;5.4
641,-2.06,0;2,-.06,0;5.4641,-.06,0;4.5981,-.56,0;4.5981,1.44,0;2.866,-1.56,0;6.3
301,1.44,0;5.4641,.94,0;2.866,-.56,0;3.7321,-.06,0;6.3301,2.06,0;6.8671,-.25,0;4
.0611,-1.87,0;6.8671,-1.87,0;5.4641,-2.68,0;2.31,.4769,0;1.4631,.25,0;1.69,-.596
9,0;
LogP values are: {'ALOGPDescriptor_ALogp2': 0.10304100000000004, 'ALOGPDescripto
r_AMR': 18.935400000000001}
Aspirin is also known as: ['2-Acetoxybenzoic acid', '50-78-2', '2-Acetoxybenzene
carboxylic acid', 'Acetylsalicylate', 'Acetylsalicylic acid', 'Aspirin', ...
'Claradin', 'Clariprin', 'Colfarit', 'Decaten', 'Dolean pH 8', ...
'Acetylsalicylsaure [German]', 'Acide acetylsalicylique [French]', ...
'A6810_SIGMA', 'Spectrum5_000740', 'CHEBI:15365',...]